Revolutionary cell separation technology
Precision microfluidic cell separation for next-generation cell therapy manufacturing
DCS (Deterministic Cell Separation) technology represents a breakthrough in cell purification for cell and gene therapies. Unlike traditional methods that rely on centrifugation or harsh chemicals, DCS uses precisely engineered microstructures to separate cells based on size with unprecedented gentleness and accuracy. Also called Deterministic Lateral Displacement (DLD), this patented technology delivers superior cell recovery while maintaining cell viability and avoiding unwanted activation - critical factors for successful cell therapy manufacturing.
This highly-scalable technology can process sample volumes from <1mL up to full-scale apheresis collections (300+ mL), generating 10’s of billions of viable, naive cells for downstream processing.
Interested in incorporating this technology into your cell and gene therapy product?
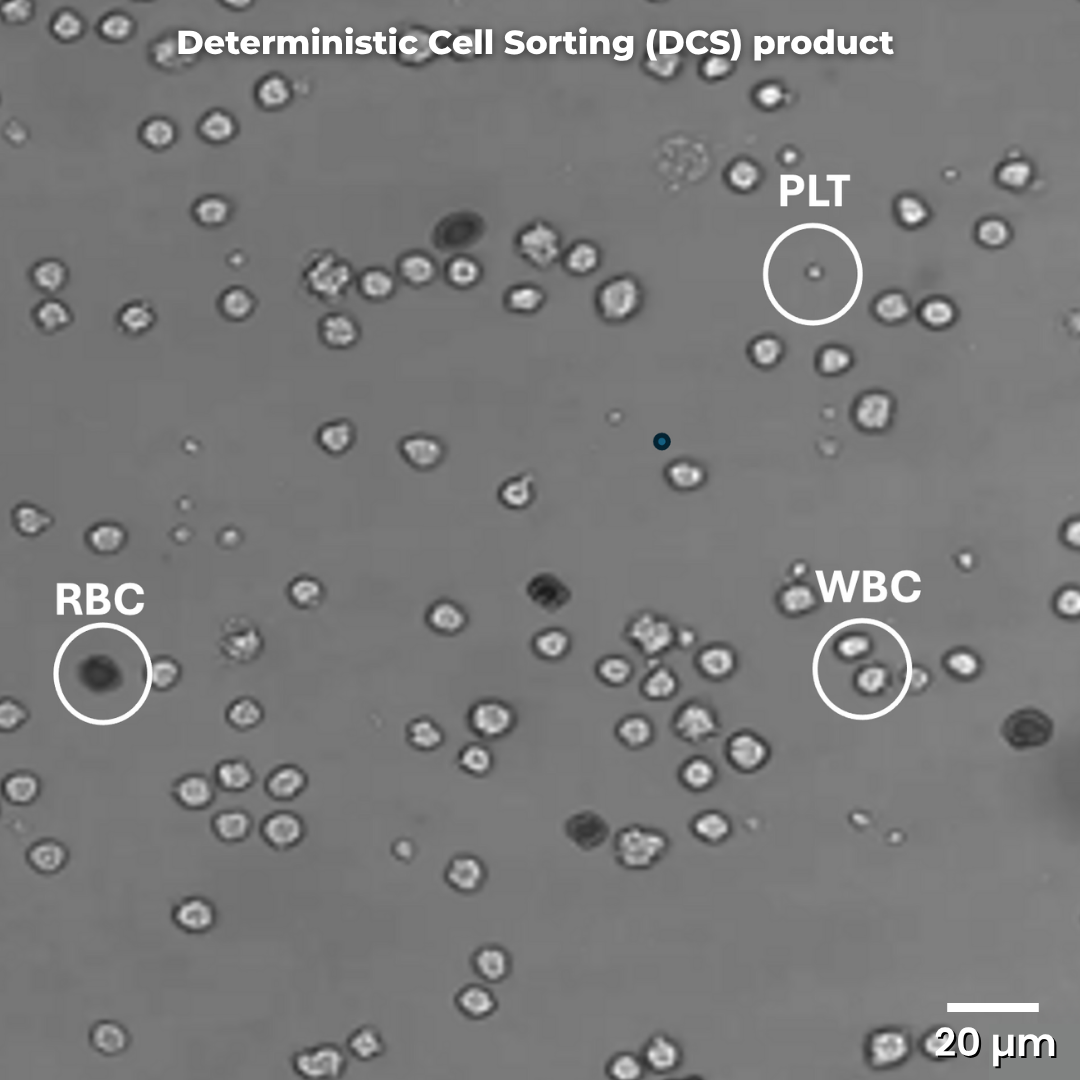
DCS purification of a leukapheresis sample shows significantly greater recovery of white blood cells (WBCs) and removal of red blood cells (RBCs) and platelets (PLTs) compared to other processes. The separation is highly reproducible and easily automated.
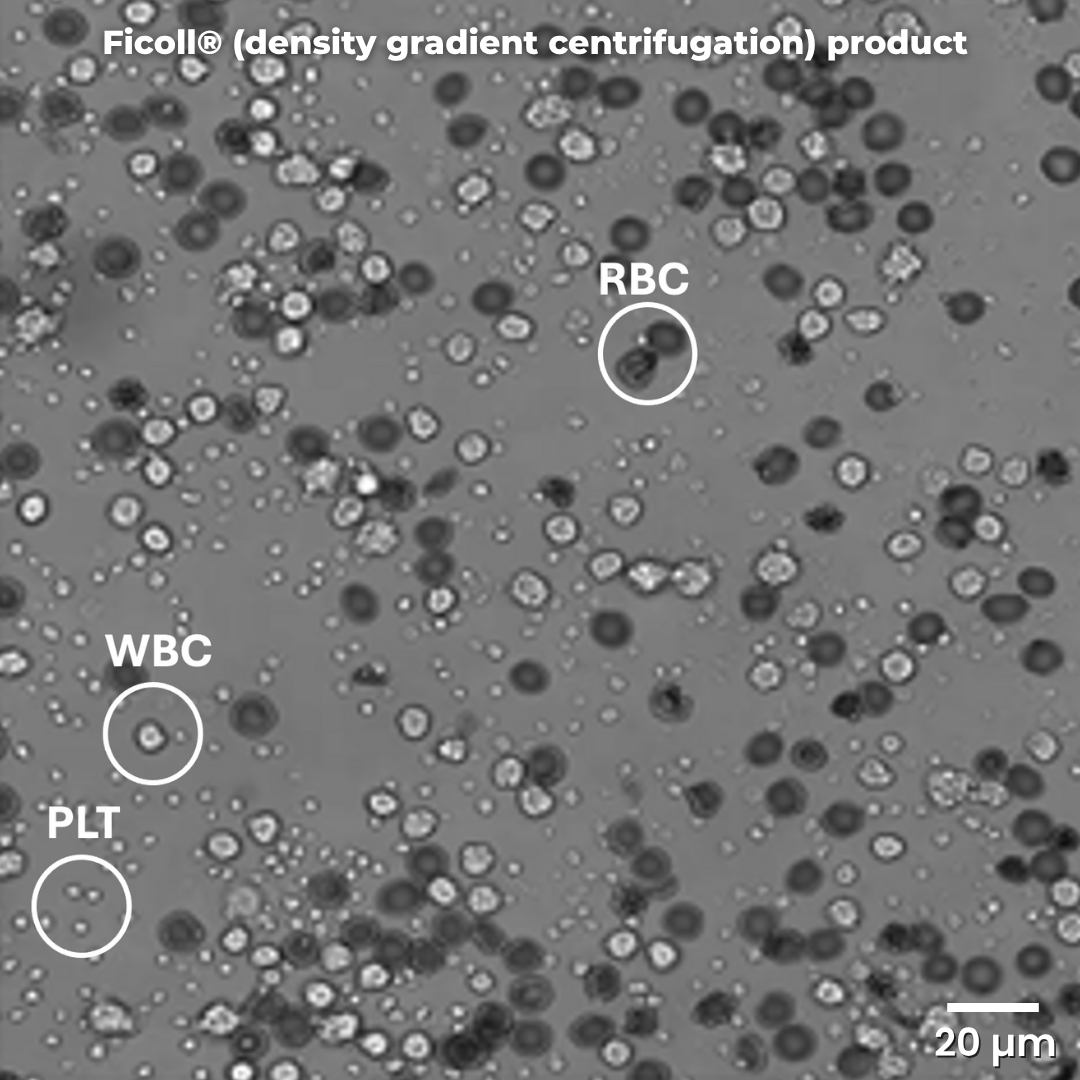
Density gradient centrifugation (Ficoll®) purification of white blood cells (WBCs) recovers fewer cells and the product is contaminated from platelets (PLTs) and red blood cells (RBCs). The process is highly variable (depends on operator) and manual.
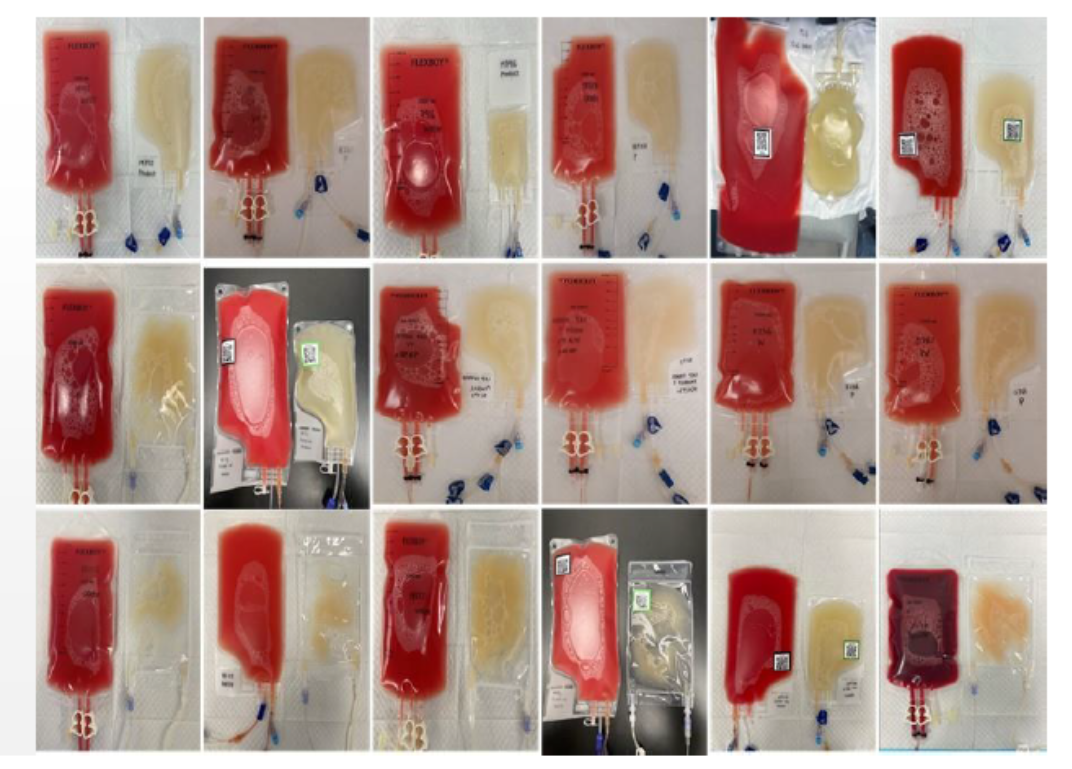
Purified WBCs products (yellow bags) and waste RBCs and PLTs (red bags) from DCS purification of a leukapheresis sample. The DCS process results in consistent WBC recovery and RBC/PLT depletion, regardless of the sample quality.
Target cells and sample types
Cell Therapy Manufacturing
- Leukocyte purification for CAR-T immunotherapy
- Stem cell purification for HSC therapies
- Cell purification prior to or post-cryopreservation
- Cell washing and concentration for QC/formulation
Sample Types
-
Fresh or frozen leukopacks
-
Mobilized leukopacks
-
Whole blood
-
Cultured/expanded target cell populations
Gentle Processing
Maintain cell viability and prevent unwanted cell activation by avoiding centrifugation or harsh chemicals.
Superior Recovery
Recover ~90% of target cells vs. 50-70% with traditional methods, enabling shorter CAR-T manufacturing processes.
Unmatched purity
Remove ~99% of PLTs and efficiently wash out soluble cytokines, yielding purified target cells in their naive state.
Mature Technology
Backed by an extensive IP portfolio and 10+ years of specialized manufacturing expertise in the CGT space.
Customizable design
Tailor arrays geometry for your specific cell populations and separation requirements.
Scalable capacity
Easily scale capacity and throughput with highly-parallelized device configurations - from 8 mL blood draws to 300 mL+ apheresis collections.
Superior performance metrics
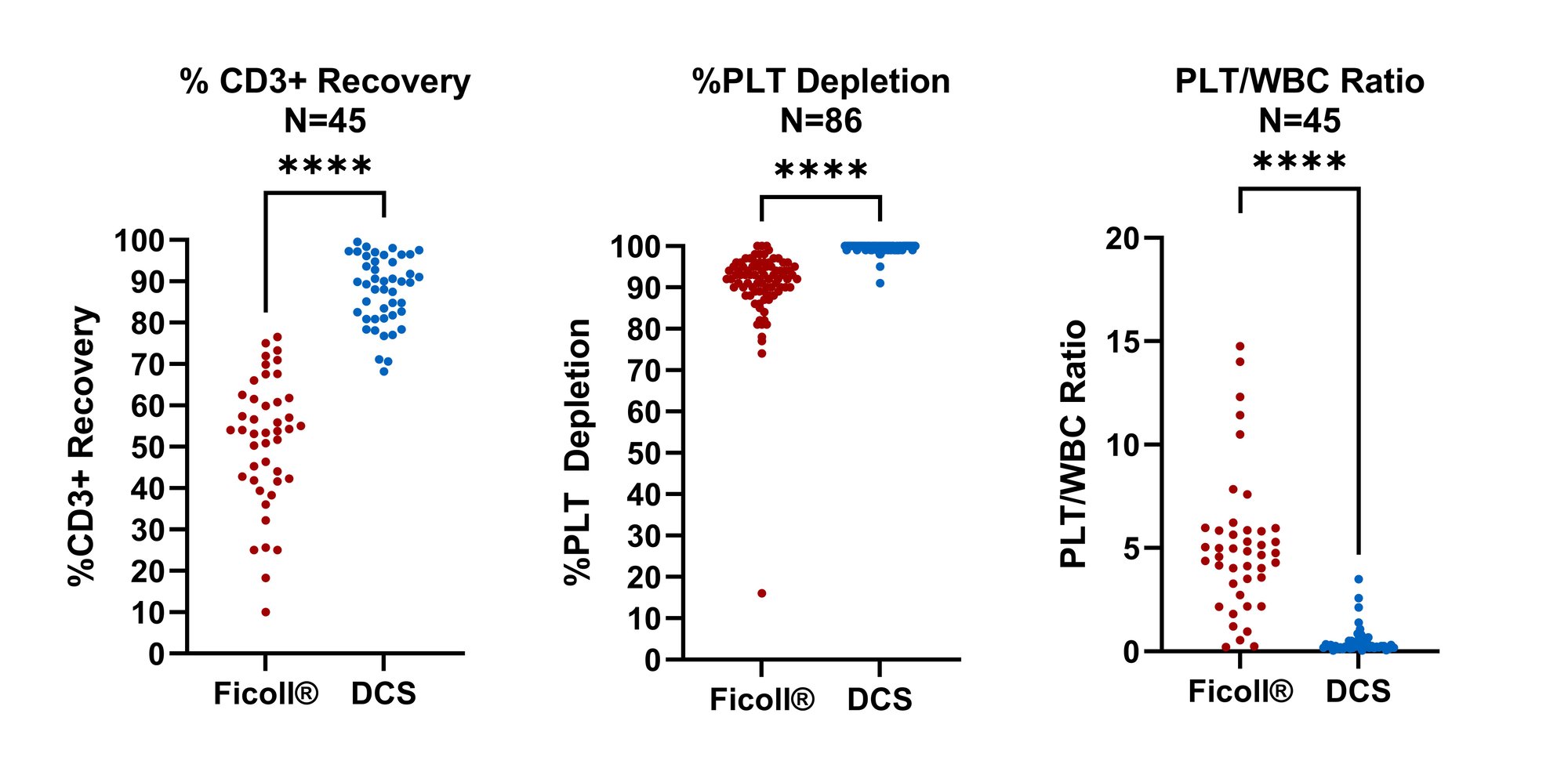
DCS recovers almost 2x more CD3+ T-cells than Ficoll®.
DCS removes >10x more platelets than manual Ficoll®.
Independent Evaluation of DCS Performance (medRxiv Preprint)
A new medRxiv preprint reports performance results from a Deterministic Cell Sorting (DCS)–based cell separation system. In a study of 150 human samples, DCS processing showed higher leukocyte recovery, improved RBC/platelet depletion, reduced T‑cell activation, better preservation of naïve/TCM subsets, and stronger expansion kinetics compared to Ficoll®.
These findings support DCS as a scalable alternative to traditional density gradient separation for CAR‑T cell manufacturing.
%20-%20Edited.png?width=810&height=797&name=Screenshot%202025-12-19%20at%2012.31.08%20PM%20(3)%20-%20Edited.png)
Technology Demonstration
Ficoll® is a registered trademark owned by GE Healthcare companies.
.png?width=152&height=65&name=a%20ZEON%20company%20(3).png)
-1.png?width=152&height=65&name=a%20ZEON%20company%20(4)-1.png)