Manufacturing of Plastic Consumables
for Diagnostic, Medical Device & Optics industries
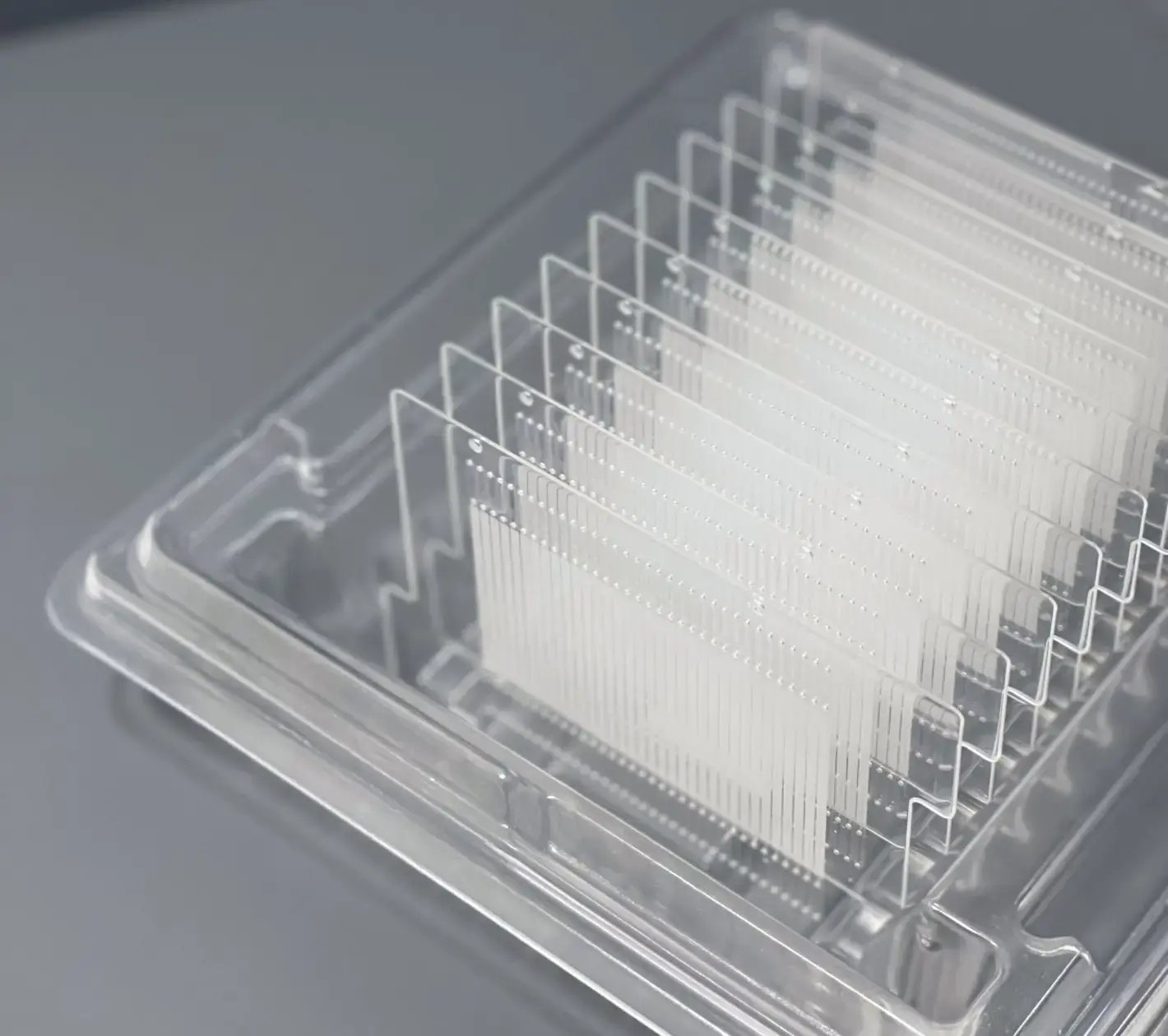
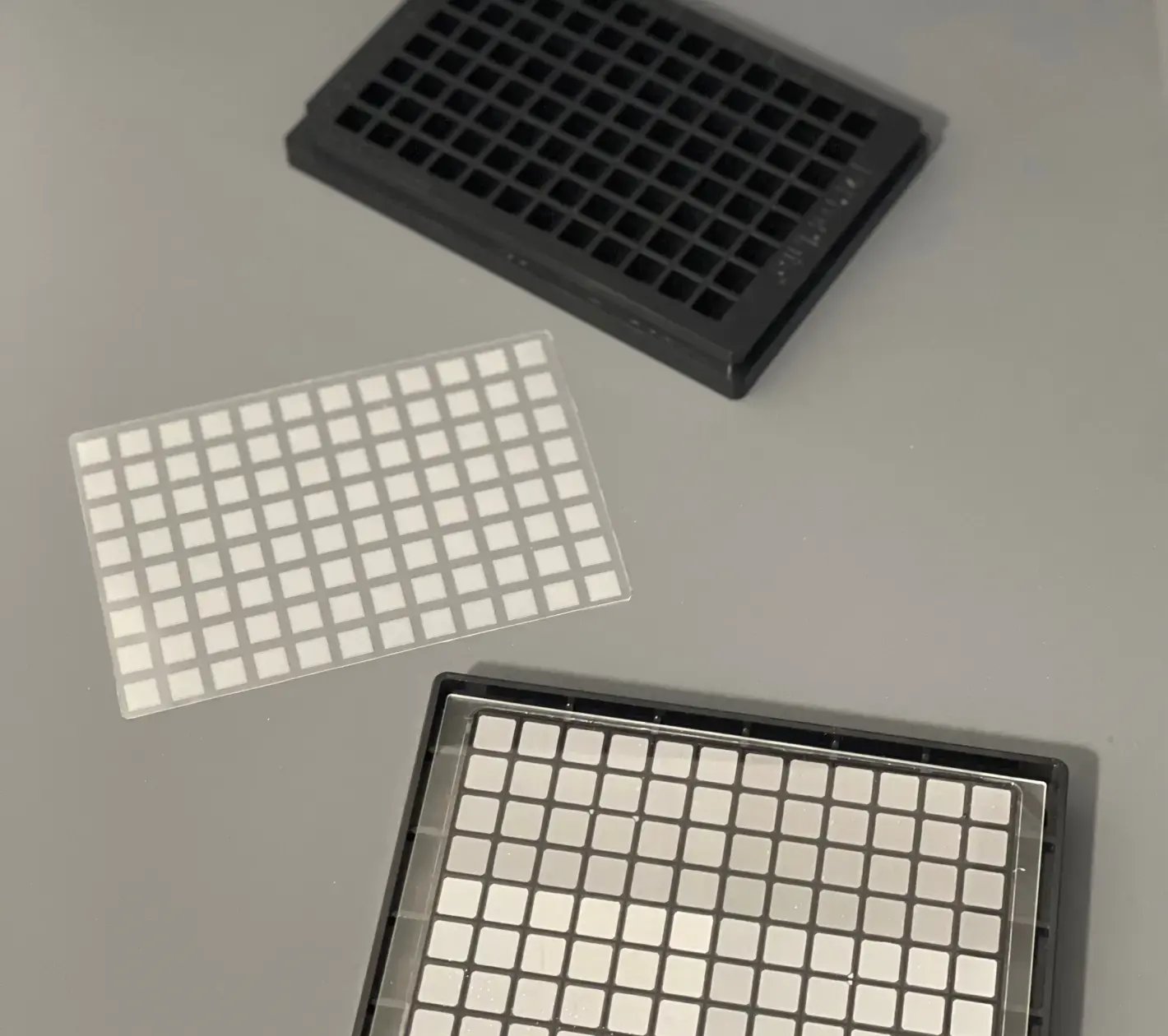
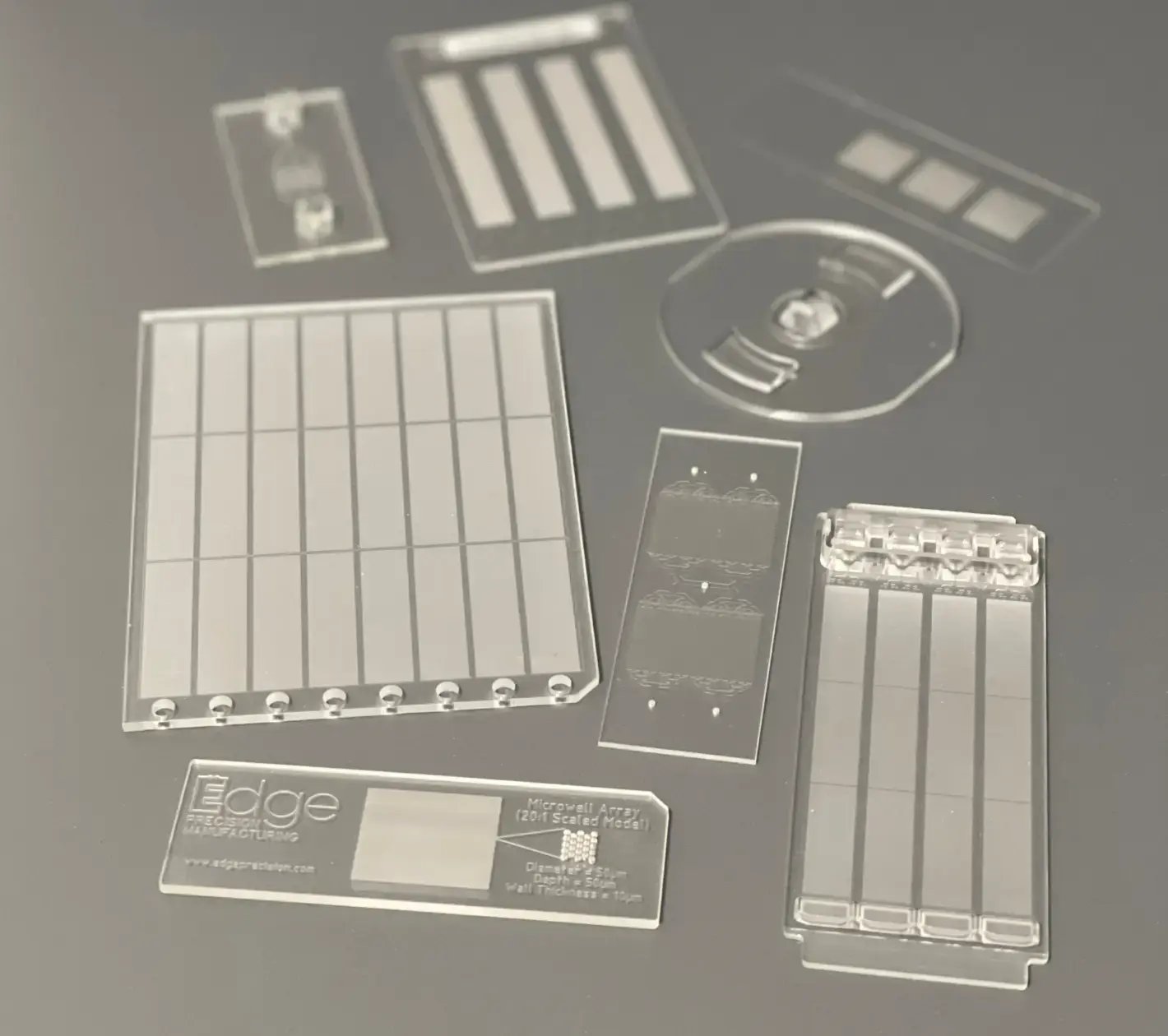

What We Can Do For You
Edge is a full-service plastic consumables manufacturer with the capabilities to take designs with precision microfeatures and tolerances from planning through production, plus handle all aspects of supply chain management.
Edge prides itself on providing the fastest path to market for high-quality products.
Compression molding 12,000 SQFT / BILLERICA MA
- ISO 13485:2016 COMPLIANT
- CLASS 7 (10,000) CLEAN ROOM
-
CAPACITY OVER 1 MILLION PIECES/YR
-
PROTOTYPES AVAILABLE FROM 1 PIECE

injection molding 48,000 SQFT - SANO JP
- ISO CERTIFIED (9001:2015, 140001:2015, 13485:2016)
- CLASS 7 (10,000) CLEAN ROOM
- CAPACITY OVER 1 MILLION PIECES/YR
- PROTOTYPES AVAILABLE FROM 1 PIECE

The Latest From Edge Precision

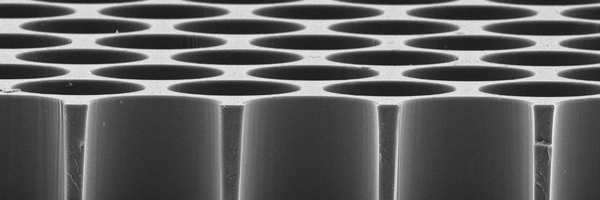
Next-generation microwell and nanowell arrays for single-cell applications
Discover how next-generation microwell and nanowell arrays enhance single-cell analysis and isolation, providing high data density and precision for diverse research applications.
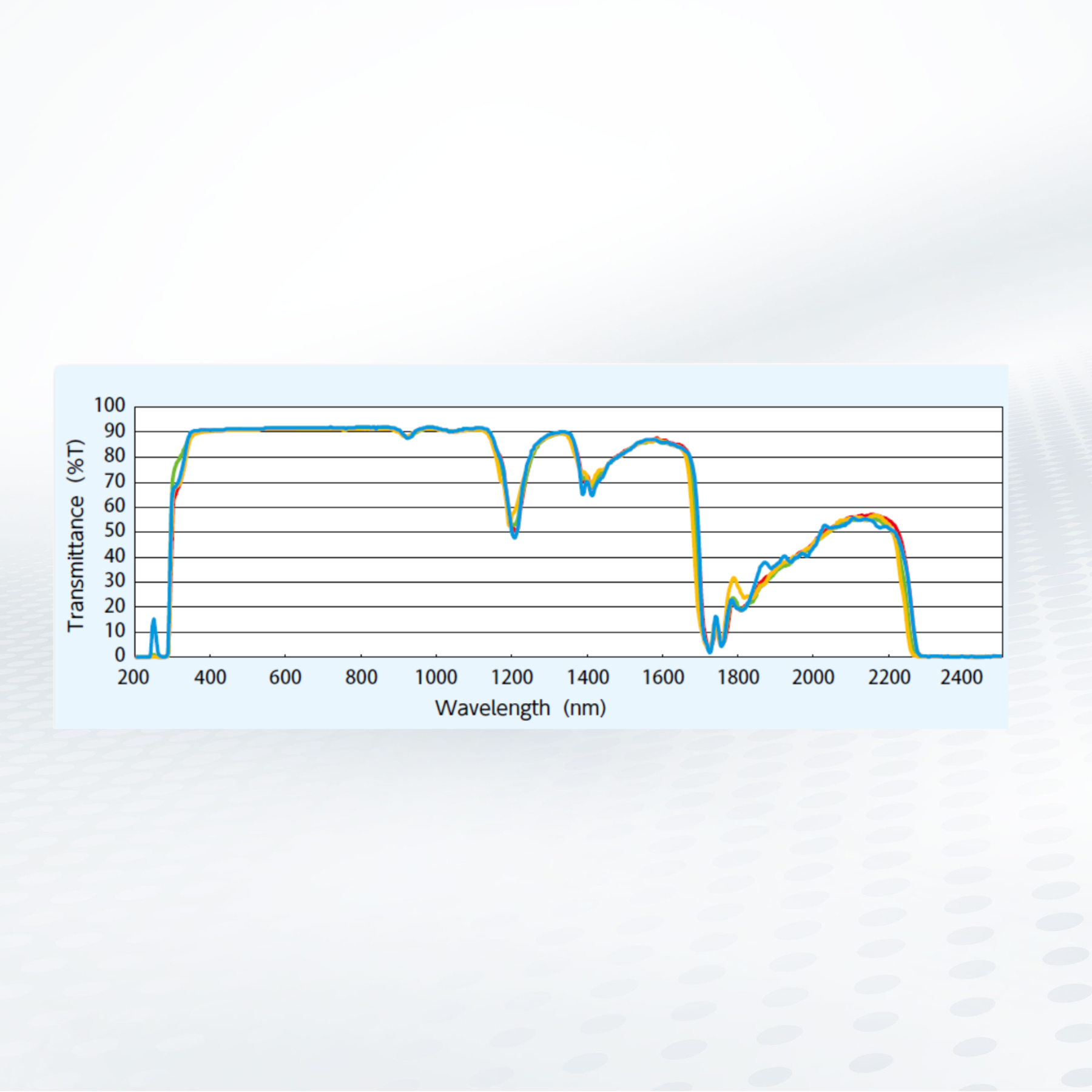
Optical Properties of Compression Molding and COP
Compression-molded Cyclo-Olefin Copolymer (COP) plastic may provide a lower-cost alternative to glass for medical, diagnostic, and optical devices.
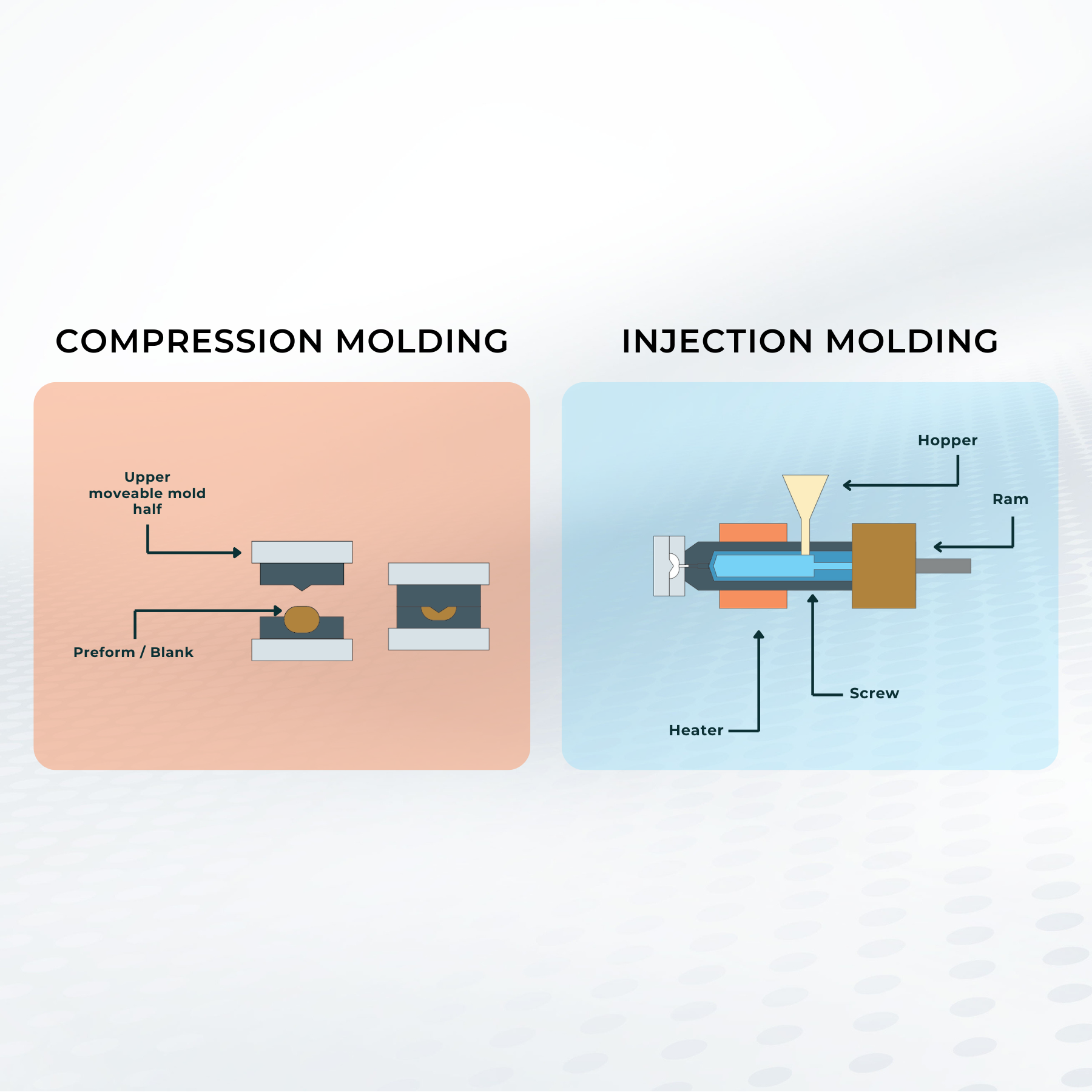
Is My Application a Good Fit for Edge's Proprietary Molding?
Custom consumable parts that offer the precision of micron-scale features at costs similar to injection molded parts, delivered in as little as 6 weeks
Additional Capabilities
As a manufacturer of precision consumable parts and assemblies, Edge has an experienced team that provides seamless service and support from design optimization through high-volume production.
Design & DFM Review
Edge’s engineers utilize our manufacturing experience to optimize your design for production scale-up and compliance with product and regulatory requirements.
Assembly
Edge's assembly process integrates robust quality controls, ensuring repeatable product manufacturing. This scalable approach guarantees consistent quality and efficiency throughout production.
Program Management
Edge provides our customers with a fast-paced collaborative experience with a focus on quality and product delivery.